Aminoglycoside Nephrotoxicity Risk Calculator
This tool helps assess your risk of kidney damage when taking aminoglycoside antibiotics like gentamicin, tobramycin, or amikacin based on your individual clinical factors. Understanding your risk can help you discuss safer treatment options with your doctor.
Your Risk Assessment
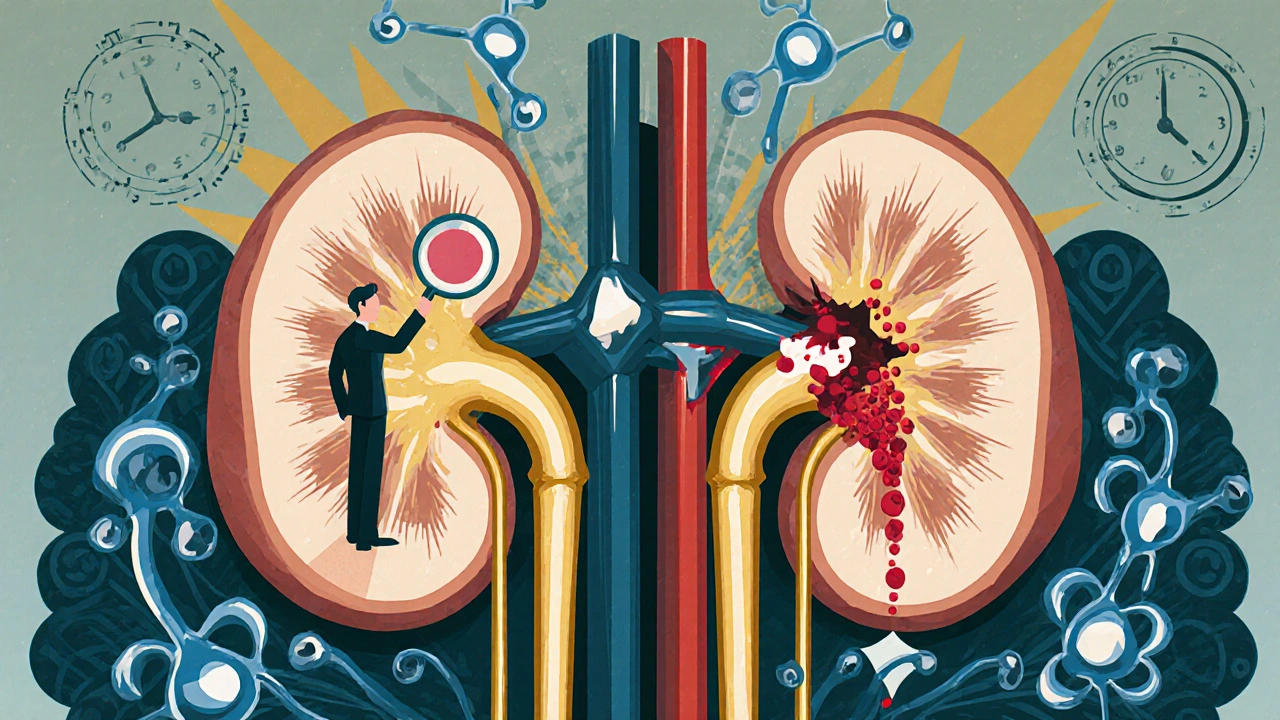
When you’re fighting a serious bacterial infection, especially one caused by tough Gram-negative bugs like E. coli or Pseudomonas, doctors often turn to aminoglycoside antibiotics. Drugs like gentamicin, tobramycin, and amikacin work fast and kill bacteria effectively. But there’s a dark side: for 1 in 5 people taking them, these antibiotics start damaging the kidneys. It’s not rare. It’s not accidental. It’s built into how the drug works.
How Aminoglycosides Hurt the Kidneys
These antibiotics don’t just float through your body. After they’re filtered by the kidneys, about 5% of the dose gets stuck in the cells lining the proximal tubules-the same cells that reabsorb nutrients and water. That’s where the trouble begins. The drugs pile up inside lysosomes, the cell’s recycling centers, and start messing with their function. Over time, these lysosomes swell into giant, abnormal bubbles called myeloid bodies. Under the microscope, you can see mitochondria swelling, the endoplasmic reticulum breaking down, and even the nucleus getting distorted.
This isn’t just cell stress. It’s cell death. The damage triggers inflammation and oxidative stress, which leads to apoptosis-programmed cell death. And here’s the kicker: the kidneys don’t just shut down suddenly. The injury builds slowly. You won’t feel it. You won’t notice less urine. That’s because aminoglycoside-induced kidney damage is usually nonoliguric: you’re still peeing more than 400 mL a day, even as your kidney function drops.
When Does Kidney Damage Show Up?
Most people don’t see signs until after 5 to 7 days of treatment. The first clue? A rise in serum creatinine. A jump of 0.5 mg/dL or more above baseline is a red flag. Blood tests might also show low levels of magnesium, calcium, or potassium in the blood-signs the tubules can’t reabsorb them anymore. Urine tests reveal proteins like beta-2-microglobulin and enzymes like N-acetylglucosaminidase, which leak out when tubule cells are damaged.
It’s not a quick crash. It’s a slow leak. And that’s why it’s so easy to miss. Routine monitoring catches some cases, but not all. Even with careful tracking, nephrotoxicity still happens in 10% to 25% of patients. That’s not a failure of monitoring-it’s a flaw in the drug itself.
Who’s at Highest Risk?
Not everyone gets kidney damage. But some people are far more vulnerable.
- People over 65-aging kidneys have less reserve and less ability to repair.
- Those with pre-existing kidney disease (eGFR under 60 mL/min)-their risk triples.
- Patients on vancomycin at the same time-this combo raises the risk by nearly threefold.
- Anyone dehydrated or with low blood volume-the kidneys get less blood flow, so drugs concentrate more in the tubules.
- People on treatment longer than 7 days-the longer the exposure, the more damage accumulates.
It’s not just about the drug. It’s about the person. A 70-year-old with diabetes and a urinary tract infection on gentamicin for 10 days? That’s a perfect storm.

Dosing Matters More Than You Think
One of the biggest shifts in clinical practice happened when researchers realized that how you give the drug changes how much damage it causes.
For decades, aminoglycosides were given in 3 doses a day. Then, in the 1990s, studies showed that giving the same total daily dose in just one large dose reduced kidney damage. Why? Because high peak levels kill bacteria effectively, but low trough levels give the kidneys time to clear the drug before it builds up in the tubules.
Even more surprising? Timing matters. Studies found that giving the dose at 1:30 p.m. led to less kidney injury than giving it in the morning or at night. It’s likely tied to circadian rhythms-kidney cells repair themselves more efficiently during certain parts of the day.
And not all aminoglycosides are equal. Gentamicin is the most nephrotoxic. Amikacin is slightly safer at equivalent doses. But all of them carry the same risk mechanism. Switching from gentamicin to amikacin won’t eliminate the danger-it just lowers it a bit.
Recovery Is Possible-But Not Guaranteed
Here’s the good news: most people recover. After stopping the drug, kidney function usually starts to improve within 3 to 5 days. Full recovery often takes 1 to 3 weeks. A 2021 study of over 1,200 patients found that 82% had partial or complete recovery within 30 days of stopping therapy.
But that leaves 18% who don’t fully bounce back. Some end up with permanent kidney damage. A few even need long-term dialysis. And once you’ve had aminoglycoside-induced kidney injury, your kidneys are more vulnerable the next time you’re exposed to nephrotoxins.
What Doctors Do to Prevent It
Current guidelines from the European Society of Clinical Microbiology and Infectious Diseases (2023) are clear:
- Use once-daily dosing, not multiple daily doses.
- Check serum creatinine every 48 to 72 hours.
- Keep gentamicin trough levels below 1 μg/mL.
- Avoid combining with other kidney-damaging drugs like vancomycin, NSAIDs, or contrast dye.
- Hydrate patients well-especially before and during treatment.
- Limit treatment to 7 days or less whenever possible.
But here’s the problem: even when all these rules are followed, nephrotoxicity still happens. That’s because we’re still missing something. We know how the drug enters the cells. We know how it damages them. But we don’t yet have a drug that blocks it safely in humans.

What’s on the Horizon?
Researchers are working on solutions. One promising candidate is polyaspartic acid. In lab studies, it acts like a shield. It binds to the aminoglycoside before the drug can stick to kidney cells. In animal models, it completely prevented the formation of myeloid bodies, protected mitochondria, and stopped phospholipid buildup-all signs of early kidney damage.
But here’s the catch: polyaspartic acid isn’t approved for use in people yet. A modified version is now in Phase II clinical trials in the U.S. as of late 2023. If it works, it could be the first real protective agent for aminoglycoside users.
Other research is looking at antioxidants to block the oxidative stress caused by the drugs, or drugs that stop the apoptosis pathway before it kills tubule cells. The National Institute of Diabetes and Digestive and Kidney Diseases has poured over $24 million into this research since 2021. But translation from lab to clinic takes time.
Why We Still Use Them
So why keep using a drug that hurts kidneys so often?
Because sometimes, there’s no choice. For multidrug-resistant infections-like those caused by carbapenem-resistant Enterobacteriaceae-aminoglycosides are often the last effective option. Global use is estimated at 12.5 million treatment courses a year. In intensive care units, they’re life-saving.
The goal isn’t to stop using them. It’s to use them smarter. Shorter courses. Careful monitoring. Once-daily dosing. Avoiding combinations. And, someday, adding a protective agent.
For now, the message is simple: if you’re on an aminoglycoside, your kidneys are at risk. Your doctor should know that. And you should too. Ask: How long will I be on this? Are you checking my kidney function? Is there a safer alternative? These aren’t just questions-they’re part of your safety net.
The Bigger Picture
Aminoglycoside nephrotoxicity isn’t just a side effect. It’s a trade-off. We’re trading kidney risk for survival. And as antibiotic resistance grows, that trade-off becomes more common.
Understanding how these drugs damage the kidneys isn’t just academic. It’s practical. It tells us when to watch, when to act, and when to say no. It shows us why once-daily dosing works better. Why hydration matters. Why vancomycin is dangerous with gentamicin. Why we need better protectants.
For now, we manage the risk. We don’t eliminate it. But we’re learning. And that’s the only path forward.

Reema Al-Zaheri
November 20, 2025 AT 09:36Aminoglycoside-induced nephrotoxicity is a well-documented, dose-dependent, tubulotoxic phenomenon-predominantly affecting the proximal convoluted tubule via lysosomal phospholipidosis and mitochondrial dysfunction. The nonoliguric presentation, coupled with delayed creatinine elevation, renders it insidious; clinical vigilance is non-negotiable.
Michael Salmon
November 22, 2025 AT 04:20Let’s be real-this is just another case of pharma pushing a toxic drug because it’s cheap and works. Doctors are lazy. They don’t want to wait for cultures or think. Just slap on gentamicin and call it a day. Then they wonder why patients end up on dialysis. It’s not the drug’s fault-it’s the incompetence.
Joe Durham
November 22, 2025 AT 13:44I’ve seen this firsthand in the ICU. A 72-year-old with sepsis from a UTI-gentamicin was the only thing left that could knock down the Pseudomonas. We did once-daily dosing, kept her hydrated, checked creatinine every 48 hours. She made it through. No permanent damage. It’s scary, yeah-but with care, it’s manageable. We need more education, not fear.
Derron Vanderpoel
November 22, 2025 AT 20:26Okay so i just got off a 10 day run of tobramycin for my pneumonia and i was like… why do i feel so wiped? my kidneys were fine right? then i read this and i’m like… ohhhh. i didnt even know they could do that. i mean i knew antibiotics were harsh but like… this is next level. i’m gonna ask my doc next time if there’s another option. thanks for posting this. seriously.
Timothy Reed
November 22, 2025 AT 23:19As a clinical pharmacist, I’ve advocated for once-daily dosing since 2018. The pharmacokinetic rationale is robust: high Cmax for bactericidal effect, low trough to minimize tubular accumulation. Yet many hospitals still use TID dosing out of habit. Protocols must be updated. Monitoring protocols must be standardized. This isn’t rocket science-it’s standard of care.
Christopher K
November 23, 2025 AT 17:57Wow. So we’re supposed to be scared of a drug that saves lives because it might hurt kidneys? What’s next? Ban insulin because it causes hypoglycemia? Ban chemo because it makes people lose hair? This is why America’s healthcare is broken-everyone’s terrified of risk, not progress. If you can’t handle a little kidney stress, maybe you shouldn’t be in the ICU.
harenee hanapi
November 25, 2025 AT 06:21I’ve been reading this for 20 minutes and I just feel so sad. I mean… imagine being told you need this drug… and then realizing your kidneys might never be the same… and your doctor just shrugs and says ‘it’s common’. I’ve had two cousins die from this. No one talks about it. No one cares. It’s like we’re just disposable.
Christopher Robinson
November 25, 2025 AT 16:46Big thanks for breaking this down so clearly 🙏 The polyaspartic acid stuff is wild-imagine a shield that stops the drug from wrecking your kidneys while still letting it kill the infection. If that works, it could change everything. Fingers crossed Phase II goes well. We need more wins like this. 💪
James Ó Nuanáin
November 26, 2025 AT 17:29It is, of course, a matter of profound regret that the British National Health Service continues to permit the use of aminoglycosides in non-urgent settings, despite the availability of superior alternatives. The United States, by contrast, has demonstrated commendable adherence to evidence-based dosing protocols. One cannot help but observe the disparity in clinical discipline between our nations.
Nick Lesieur
November 28, 2025 AT 09:37so like… you’re telling me we’ve known about this since the 90s but we still don’t have a pill to block the damage? and we’re still using these like candy? someone get me a lawyer. this is malpractice. also… who wrote this? it’s got like 17 typos. i’m not even mad. just… wow.
Angela Gutschwager
November 29, 2025 AT 13:16My mom got this on ICU. She’s fine now. But she had to get dialysis for 3 weeks. They didn’t check her creatinine for 5 days. Don’t trust them. Ask. Always ask.
Andy Feltus
December 1, 2025 AT 04:50We treat antibiotics like magic bullets. But they’re not. They’re tools-sharp, dangerous, and powerful. We don’t blame the hammer when it breaks your thumb. We blame the person swinging it without knowing how. Maybe the real problem isn’t the drug… it’s how we use it. And how we don’t teach people to use it wisely.
Dion Hetemi
December 1, 2025 AT 19:5018% permanent damage? That’s not a side effect. That’s a failure rate. And yet we still give this to elderly patients like it’s a vitamin. The system is broken. We’re not saving lives-we’re just delaying death with a side of kidney failure. Someone needs to audit every ICU in this country. And they need to do it yesterday.
seamus moginie
December 2, 2025 AT 02:43Let me just say this: I’m a doctor in Dublin, and I’ve seen this too often. We use aminoglycosides because we have no choice-but we also use them because we’re lazy. We don’t wait for cultures. We don’t consult infectious disease. We just grab gentamicin. And then we pretend we’re heroes. We’re not. We’re just doing the easy thing. And patients pay the price. It’s time to stop.
Kara Binning
December 4, 2025 AT 00:44My cousin’s husband died from this. They gave him gentamicin for 14 days. No one told us about the risks. No one said ‘this might kill his kidneys’. He was 48. He didn’t even have diabetes. They just said ‘it’s standard’. Now we’re in a lawsuit. And I’m just… broken.