Digital diagnostic technologies is a collection of electronic tools that enable faster, more accurate detection and monitoring of pulmonary tuberculosis, characterized by rapid molecular assays, AI‑driven imaging, and mobile health platforms.
Why technology matters in the fight against TB
Pulmonary tuberculosis (TB) still kills over 1.5 million people each year, according to the World Health Organization. The biggest challenge isn’t the bacteria itself - it’s finding the infection early enough to treat it before it spreads. Traditional sputum smear microscopy, while cheap, misses up to 50% of cases, especially in people living with HIV. Modern technology closes that gap by delivering results in hours instead of weeks and by reaching patients in remote clinics.
Key technological pillars
The landscape can be grouped into three pillars: rapid molecular testing, AI‑augmented imaging, and digital patient‑centred care. Each pillar links back to the central goal - a quicker, more reliable diagnosis and a smoother treatment journey.
Rapid molecular testing
GeneXpert MTB/RIF is a cartridge‑based nucleic acid amplification test that detects Mycobacterium tuberculosis DNA and rifampicin resistance in under two hours. Its sensitivity (>95%) and specificity (>98%) far outperform smear microscopy, making it the gold standard for point‑of‑care diagnosis in many high‑burden countries.
Other molecular platforms, such as Truenat and Xpert Ultra, extend the same principle to smaller labs with lower power needs, widening geographic coverage.
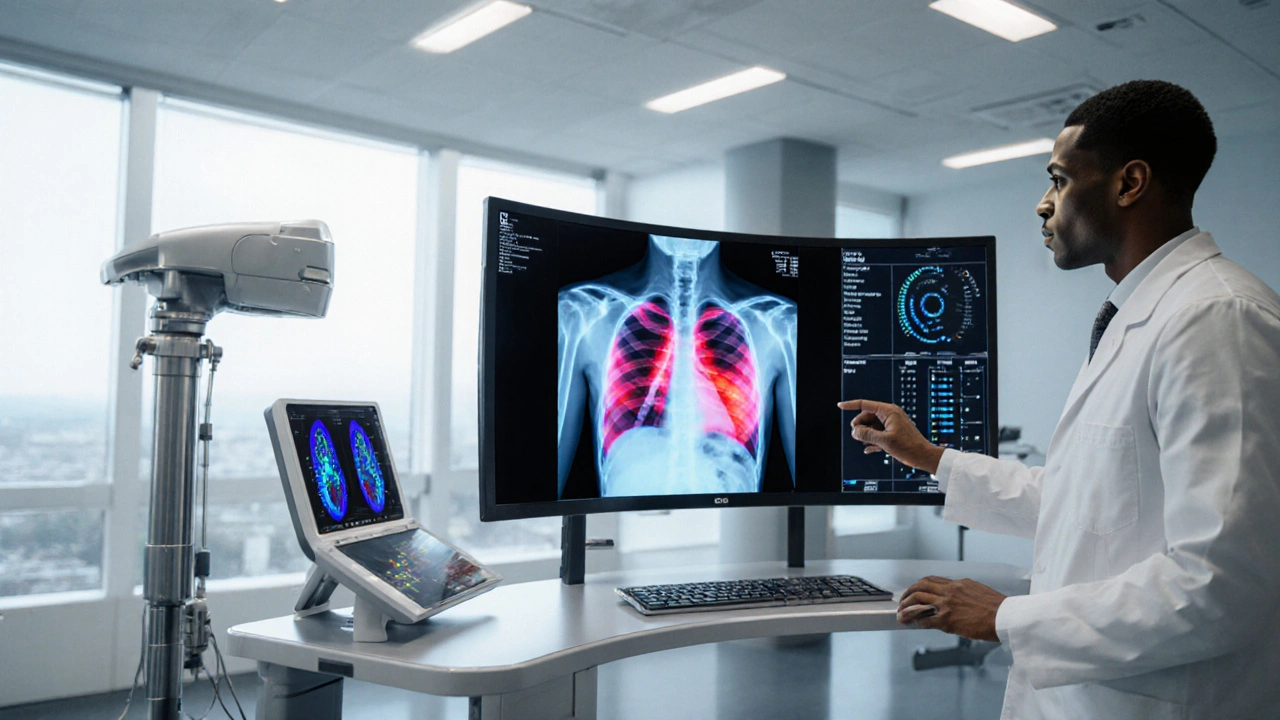
AI‑driven chest imaging
AI chest X‑ray analysis is a software system that uses deep‑learning algorithms to spot TB‑related abnormalities on digital radiographs. In a 2022 multi‑centre study, AI tools achieved a sensitivity of 93% and specificity of 89% compared with human radiologists, cutting interpretation time from minutes to seconds.
This technology matters most in settings where radiologists are scarce. A mobile van equipped with a digital X‑ray machine and AI software can screen thousands of people in a day, instantly flagging suspicious scans for follow‑up testing.
Digital patient‑centred care
mHealth apps are smartphone applications that support TB patients with medication reminders, side‑effect reporting, and direct communication with health workers.
When combined with telemedicine platforms, these apps enable remote adherence monitoring, reducing default rates by up to 30% in a 2023 trial across Kenya and Ethiopia.
Comparison of leading diagnostic technologies
| Technology | Turn‑around time | Sensitivity | Infrastructure needed |
|---|---|---|---|
| GeneXpert MTB/RIF | 2hours | 95% | Desktop module, stable electricity |
| AI chest X‑ray | Seconds (image upload) | 93% | Digital X‑ray + internet |
| Whole‑Genome Sequencing | 24‑48hours | 99% (for strain typing) | Sequencer, bioinformatics pipeline |
How these tools fit into the WHO End TB Strategy
The World Health Organization’s End TB Strategy calls for “universal access to rapid, accurate diagnostics”. Digital technologies meet that call in three ways:
- Early case detection - rapid molecular tests uncover both drug‑sensitive and drug‑resistant TB within hours.
- Integrated care pathways - AI imaging alerts health workers instantly, prompting same‑day confirmatory testing.
- Patient‑centred monitoring - mHealth tools keep patients engaged throughout the six‑month regimen.
Implementation studies in India, South Africa, and Brazil show that when at least two of these pillars are combined, treatment initiation time drops from a median of 21days to under three days.

Challenges and safeguards
Technology is not a silver bullet. Data privacy, equipment maintenance, and staff training are recurring hurdles. For instance, AI models trained on high‑resolution X‑rays from tertiary hospitals may under‑perform in rural clinics with lower image quality. Ongoing calibration and local validation are essential.
Regulatory frameworks such as the EU’s In‑Vitro Diagnostic Regulation (IVDR) and WHO’s Prequalification Programme provide quality assurance, but low‑resource settings often lack the capacity to navigate them. Partnerships with NGOs and public‑private initiatives can bridge that gap.
Future directions
Three trends are shaping the next decade:
- Point‑of‑care genomics - portable sequencers could identify drug‑resistance mutations on the spot, enabling personalized therapy.
- Integrated health information systems - linking EHRs, laboratory data, and mHealth logs creates a single patient timeline, improving cohort monitoring.
- Predictive analytics - machine‑learning models that forecast outbreak hotspots help allocate resources before cases surge.
When these innovations converge, the vision of a TB‑free world becomes tangible.
Putting technology into practice - a quick‑start checklist
- Assess existing laboratory capacity - is a GeneXpert module feasible?
- Secure reliable internet for AI imaging uploads.
- Train frontline workers on sample collection and data entry.
- Choose an mHealth platform that complies with local privacy laws.
- Set up a monitoring dashboard that pulls data from diagnostics, EHR, and patient‑reported outcomes.
Frequently Asked Questions
How fast can GeneXpert detect TB compared to a smear test?
GeneXpert delivers a result in about two hours, while a sputum smear can take several days to process and often misses half of the true cases.
Can AI chest X‑ray replace a radiologist?
AI assists rather than replaces radiologists. It flags suspicious images instantly, allowing a human expert to focus on confirmation and treatment decisions.
What are the costs of implementing these technologies in a low‑resource clinic?
A GeneXpert module costs roughly $17,000 plus per‑cartridge fees (~$10 each). Mobile X‑ray units with AI software run about $50,000 upfront, but a subscription model can spread the expense. mHealth apps often have minimal fees, especially when supported by NGOs.
How does whole‑genome sequencing help TB management?
Sequencing reveals the exact drug‑resistance mutations, guiding clinicians to choose the most effective regimen, which reduces treatment failure and transmission of resistant strains.
Are there privacy concerns with mHealth apps for TB patients?
Yes. Apps must encrypt data, store it securely, and obtain informed consent. Many national TB programs now follow WHO’s digital health guidelines to safeguard patient information.

Laneeka Mcrae
September 27, 2025 AT 16:07GeneXpert has basically turned TB testing into a coffee‑break activity – two hours and you have a result, not a week‑long mystery. The cartridge system also tells you if rifampicin resistance is present, which is a huge step for starting the right regimen early. AI chest X‑rays take the guesswork out of radiology in places where a specialist is a luxury, flagging suspicious scans in seconds. Mobile apps keep patients on track with reminders and side‑effect reporting, cutting default rates noticeably. All these tools together shrink the diagnostic window and help hit the WHO End TB targets faster.
Kendra Barnett
October 10, 2025 AT 13:56Seeing these digital tools in action really gives me hope for the communities that struggle with TB. When a nurse can send an X‑ray to an AI cloud and get a readout instantly, it feels like a superpower. The reminder apps also mean patients aren't left alone during the long treatment course. Keep sharing these success stories – they motivate more clinics to adopt the tech.
Warren Nelson
October 23, 2025 AT 11:45I've been following the rollout of portable sequencers in a few African labs – the speed is wild. Even though the hardware still needs a stable power source, getting resistance data on the same day changes how doctors prescribe meds. It's a chill reminder that tech can fit into low‑resource settings when we think creatively.
Jennifer Romand
November 5, 2025 AT 08:34One could argue that the hype around AI in radiology eclipses the nuanced art of clinical judgment. Yet, the technology merely amplifies, not replaces, the seasoned eye.
Kelly kordeiro
November 18, 2025 AT 06:23It is incumbent upon us, as custodians of public health, to scrutinize the tapestry of technological interventions that promise to revolutionize pulmonary tuberculosis management. While the allure of rapid molecular assays such as GeneXpert is undeniable, one must not overlook the infrastructural exigencies that accompany their deployment – stable electricity, routine cartridge supply chains, and calibrated maintenance protocols. Equally, the seductive promise of artificial intelligence in chest imaging, though empirically validated with sensitivities approaching ninety‑three percent, demands an ecosystem of high‑resolution digital radiography and robust broadband connectivity, lest its performance degrade in austere environments. The burgeoning domain of mobile health applications, lauded for their adherence‑enhancing reminders, introduces a labyrinth of data‑privacy conundrums; encryption standards, informed consent mechanisms, and compliance with nascent regulatory frameworks must be meticulously orchestrated. Moreover, the specter of inequity looms large: communities bereft of digital literacy may find themselves disenfranchised by tools they cannot navigate. To mitigate such disparities, capacity‑building initiatives-ranging from on‑site technician training to community outreach-must be embedded within each implementation schema. Furthermore, the integration of whole‑genome sequencing, with its unparalleled resolution of resistance mutations, heralds a new epoch of personalized therapeutics, yet its financial and logistical burdens are formidable, necessitating innovative financing models. The confluence of these modalities, when harmonized within a unified health information system, engenders a comprehensive patient journey from detection to cure. Predictive analytics, leveraging machine‑learning algorithms on aggregated epidemiological data, can preemptively flag outbreak hotspots, thereby optimizing resource allocation. Nevertheless, the promise of point‑of‑care genomics, while tantalizing, remains tethered to the realities of sample preparation and bioinformatic expertise. In sum, the trajectory toward an TB‑free world is contingent upon a symphony of technology, policy, and human ingenuity, each bearing its own cadence and constraints.
Chris Fulmer
December 1, 2025 AT 04:12The integration of these tools into existing health systems is where the rubber meets the road. I've seen pilot programs where health workers use a single tablet for both AI‑assisted X‑rays and mHealth reminders, and the workflow becomes surprisingly smooth. The key is local validation – ensuring the AI model works on the imaging hardware available in the field. If that foundation is solid, scaling becomes far less intimidating.
William Pitt
December 14, 2025 AT 02:01Building on the previous point, we should prioritize interoperable platforms that let GeneXpert results flow directly into the same dashboard that hosts AI image reads and patient‑reported outcomes. When a clinician sees a positive molecular test, an AI‑flagged chest X‑ray, and a reminder‑compliance score in one glance, decision‑making accelerates dramatically. This cross‑talk between technologies also helps catch inconsistencies – a negative X‑ray in the face of a strong molecular signal could trigger a retest. Moreover, training modules that cover the entire digital suite rather than isolated tools foster a more confident health workforce. Finally, partnerships with telecom providers can secure the bandwidth needed for real‑time image uploads, keeping the whole system humming.
Jeff Hershberger
December 26, 2025 AT 23:50Tech wins.
Jesse Najarro
January 8, 2026 AT 21:39Nice but needs funding you know i think more gov support would help yeah
Dan Dawson
January 21, 2026 AT 19:28Funding is key it drives roll‑out and maintenance
Lawrence Jones II
February 3, 2026 AT 17:17🚀 Absolutely, leveraging interoperable APIs and cloud‑native pipelines can democratize data access 📊. Think about real‑time dashboards powered by FHIR standards 🌐, syncing GeneXpert outputs, AI scores, and mHealth adherence metrics. This orchestration reduces latency and boosts clinical decision support 💡. Plus, containerized deployments ensure scalability across variable bandwidth environments. In short, the tech stack must be as agile as the pathogens we’re fighting.